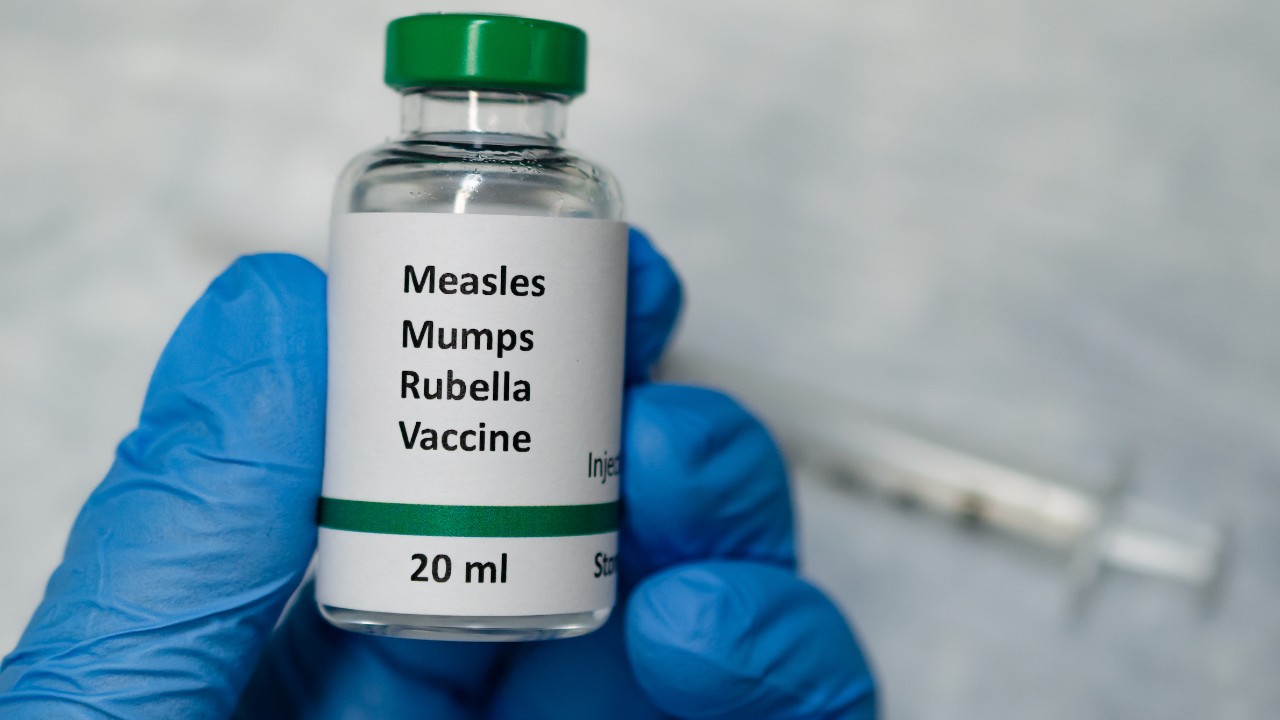
DiYES International School – MMRV vaccines unexpectedly became the focal point of a chaotic two-day meeting led by advisers to the US Centers for Disease Control and Prevention. What began as a planned discussion around altering the timeline for the newborn hepatitis B vaccine quickly spiraled into procedural confusion and a delay in voting. The committee initially intended to shift the administration of the hepatitis B vaccine from immediately after birth to one month of age.
However, debates grew intense as concerns surfaced about whether this change might undo decades of progress in hepatitis B prevention. Hepatitis B, once infecting nearly 18000 newborns annually in the US, has been largely controlled due to early vaccination. But Thursday’s meeting ended in confusion, with only a single vote cast to delay further decision-making. This left the vaccine schedule in limbo, setting the stage for Friday’s renewed focus on a different but equally important issue: the combined MMRV vaccines for children.
As confusion from the previous day carried over, MMRV vaccines dominated Friday’s discussion. The committee revisited its earlier controversial vote against including the MMRV shot in the Vaccines for Children program. On Thursday, the recommendation against MMRV for young children startled both healthcare providers and policy advocates. Dr Jason Goldman, a liaison to the committee, pointed out that such a decision might result in unequal access to vaccines, especially among lower-income families. On Friday, however, ACIP members shifted course, voting 9-0 with three abstentions to include the MMRV shot in the program.
The reversal aligned CDC policy with expert consensus and addressed fears about creating two vaccine standards based on socioeconomic status. Although some members expressed concerns about adverse events following MMRV vaccination, data reviewed by the committee suggested those risks remain extremely rare. The final decision on implementation now rests with the CDC’s acting director and the Department of Health and Human Services.
The vaccine debate did not unfold in a vacuum. The MMRV vaccines discussion and the hepatitis B delay came at a moment of internal turmoil within the CDC. Just weeks prior, Dr Susan Monarez was removed from her position as agency head without public explanation. Jim ONeill, a deputy under Health and Human Services Secretary Robert Kennedy Jr, now serves as acting CDC director. This leadership shuffle introduced additional tension into an already controversial process.
Several members of the advisory group appeared unsure whether their votes would carry immediate policy implications or face political scrutiny. One anonymous official hinted that ONeill’s stance on childhood vaccinations remains unclear. This uncertainty contributed to the chaotic nature of the two-day meeting, with procedural errors, conflicting motions and frustrated advisers. While the CDC maintains that its recommendations are evidence-based, critics fear political interference could erode public trust in childhood vaccine programs. As more voices call for transparency, the agency faces growing pressure to stabilize its internal leadership.
“Read more: Did Sylvester Stallone Just Leak a Shocking Villain Twist in Tulsa King Season 3?”
Outside the agency, pediatricians and public health experts raised alarm over the delayed hepatitis B vote. Many feared that postponing changes might send mixed messages to hospitals and parents. The birth dose of the hepatitis B vaccine has been a cornerstone of early childhood protection in the United States for over three decades. Since its widespread adoption, infant cases of hepatitis B have dropped from tens of thousands to only a few dozen each year.
Children who contract hepatitis B face a near-certain risk of developing chronic infections, which can lead to liver damage, cirrhosis or cancer. Some advocacy groups accused the committee of downplaying these risks by entertaining schedule changes without full consensus. Hospital administrators also warned that inconsistency in CDC guidance could cause logistical confusion for birth centers. While the decision has not yet been finalized, this week’s events made clear that vaccine policy decisions remain both highly technical and deeply political in nature.
Looking ahead, the CDC must balance clinical evidence with ethical considerations as it finalizes positions on MMRV vaccines and other childhood immunizations. The MMRV debate brought forward concerns about equal access, data transparency and the possibility of two-tiered healthcare. At the same time, the delayed hepatitis B vote highlighted the fragility of scientific consensus when procedural chaos dominates. With acting director Jim ONeill now at the helm and HHS reviewing all recommendations, some worry that future decisions may reflect political strategy rather than purely medical advice. Public confidence in the CDC has already been tested by recent controversies surrounding COVID-19, vaccine mandates and public health messaging. This latest episode adds fuel to ongoing debates about the agency’s independence and credibility. For now, families and providers await clarity, hoping that science—not confusion—guides the next steps in America’s vaccine strategy.
This article is sourced from edition.cnn.com and for more details you can read at diyesinternational
Writer: Sarah Azhari
Editor: Anisa