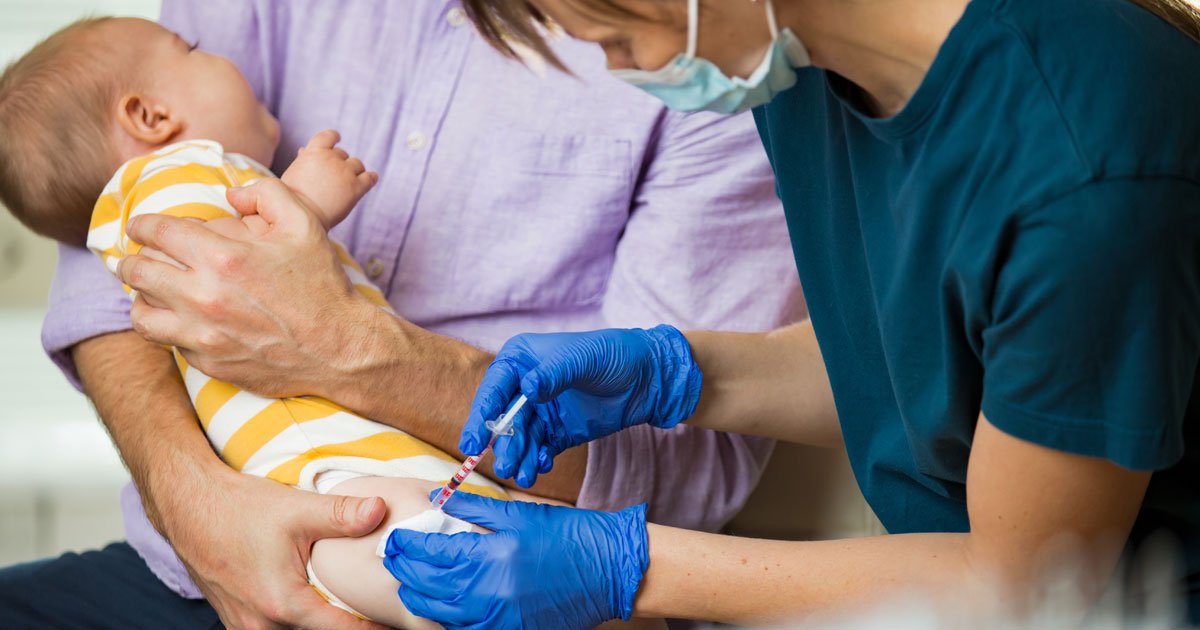
DiYES International School – Clesrovimab Gains FDA Approval to protect vulnerable infants from respiratory syncytial virus or RSV during their first exposure season. This groundbreaking antibody treatment, also known as Enflonsia, received authorization after strong evidence showed that it reduces hospitalization risk significantly. In large-scale clinical trials, infants who received a single injection of clesrovimab experienced up to a 91 percent reduction in RSV-related hospitalizations. RSV is one of the most common causes of lower respiratory tract illness in infants and young children, often leading to severe lung inflammation and difficulty breathing. With approval granted by the United States Food and Drug Administration, healthcare providers across the country will now begin preparing for widespread distribution of this preventive option before peak RSV season. Experts believe this step could be a turning point in pediatric health, especially in areas where healthcare systems often struggle during seasonal respiratory surges.
Clesrovimab Gains FDA Approval after demonstrating strong, consistent outcomes in protecting infants from serious RSV complications. Research teams collaborated globally to test the drug across diverse infant populations, evaluating safety and efficacy over multiple seasons. A randomized controlled trial found that babies given clesrovimab had hospitalization rates dramatically lower than those without protection. Medical professionals closely monitored participants for any adverse effects, and tolerability remained high. The treatment uses a laboratory-generated monoclonal antibody that targets the RSV virus directly, preventing it from replicating inside the body. Unlike a vaccine, clesrovimab works immediately, offering passive immunity for several months. Regulatory experts reviewed trial data and confirmed that benefits outweighed any minor risks. Approval was granted under pediatric safety regulations that prioritize rapid access for life-saving therapies. The breakthrough comes at a critical time, as RSV continues to be one of the top causes of hospitalization for children under two years of age worldwide.
“Read about: Dr Krishnan Leads Historic Surgery on Newborn”
Clesrovimab is expected to create a meaningful public health shift in how countries respond to seasonal RSV outbreaks. With an 84 to 91 percent reduction in hospitalization risk, the drug brings hope to millions of families each year. Pediatric emergency rooms often become overwhelmed during RSV season, straining healthcare capacity in urban and rural areas alike. The approval of clesrovimab will ease this pressure significantly. Health economists have predicted that widespread use could result in fewer emergency visits, shorter hospital stays, and substantial cost savings for both public and private systems. Additionally, reduced RSV burden can improve mental health outcomes for caregivers who previously had limited preventive tools. Medical guidelines are now being updated to include clesrovimab for high-risk infants, especially those born prematurely or with underlying lung conditions. Broader immunization access can also help close equity gaps in pediatric care across socio-economic groups.
Public health agencies and pediatricians are now working to integrate clesrovimab into national infant immunization schedules. Implementation plans include public education campaigns, updated provider protocols, and financial subsidies for underserved families. Because the treatment requires only one dose per season, it is more accessible than traditional multi-dose vaccine series. Clesrovimab can be administered at hospitals, pediatric clinics, or birth centers before infants leave medical care for the first time. Medical supply chains are preparing for large-scale distribution ahead of autumn, the typical peak for RSV cases. Health officials stress that early deployment will be critical to reduce the virus’s impact this year. In low-resource settings, partnerships with NGOs will support cold chain management and equitable allocation. The drug’s inclusion in routine infant care represents a leap forward in reducing preventable respiratory illness, and it sets the stage for other antibody-based interventions in early-childhood disease prevention.
“Read more: Walking for Her: A Journey of Hope Across Three States”
Clesrovimab’s success has opened new possibilities for monoclonal antibodies in pediatric care beyond RSV prevention. Drug developers are now exploring similar treatments for other seasonal infections like influenza and emerging respiratory viruses. Unlike traditional vaccines that require immune system training, monoclonal antibodies provide immediate protection, making them ideal for infants with underdeveloped immunity. Research groups are investigating how these drugs can support neonatal intensive care units and improve survival rates among preterm babies. Clesrovimab also demonstrates the effectiveness of accelerated approval pathways when data is clear and risk is minimal. With increased funding, more biotech companies may invest in next-generation antibody therapies tailored to child health. This shift could lead to an entirely new category of pediatric interventions, where customized biologics play a central role in disease prevention. By setting a precedent, clesrovimab may become the first in a long line of protective treatments that redefine early medical care for children.